


This article examines the pivotal role of ANVISA, Brazil's regulatory authority for clinical trials, in shaping the design and execution of clinical research studies. It underscores that ANVISA enforces rigorous guidelines and requirements, including the necessity for comprehensive clinical trial applications and strict adherence to ethical practices. These measures not only safeguard participant safety and ensure compliance but also cultivate an environment that promotes innovative medical research.
In the dynamic landscape of clinical research, Brazil's regulatory framework is pivotal in shaping trial conduct, ensuring that innovation aligns with stringent safety and ethical standards. At the forefront of this regulatory oversight is ANVISA, the Brazilian Health Regulatory Agency, tasked with the critical mission of safeguarding public health while facilitating the advancement of medical technology. Recent legislative changes and updates to regulatory guidelines present researchers with both opportunities and challenges in navigating this complex environment.
From expedited approval processes to the necessity of comprehensive ethical reviews, understanding ANVISA's requirements is essential for successful clinical trials. As Brazil positions itself as a burgeoning hub for medical research, insights into ANVISA's evolving role and the implications of these regulations will be invaluable for stakeholders aiming to drive innovation in the healthcare sector.
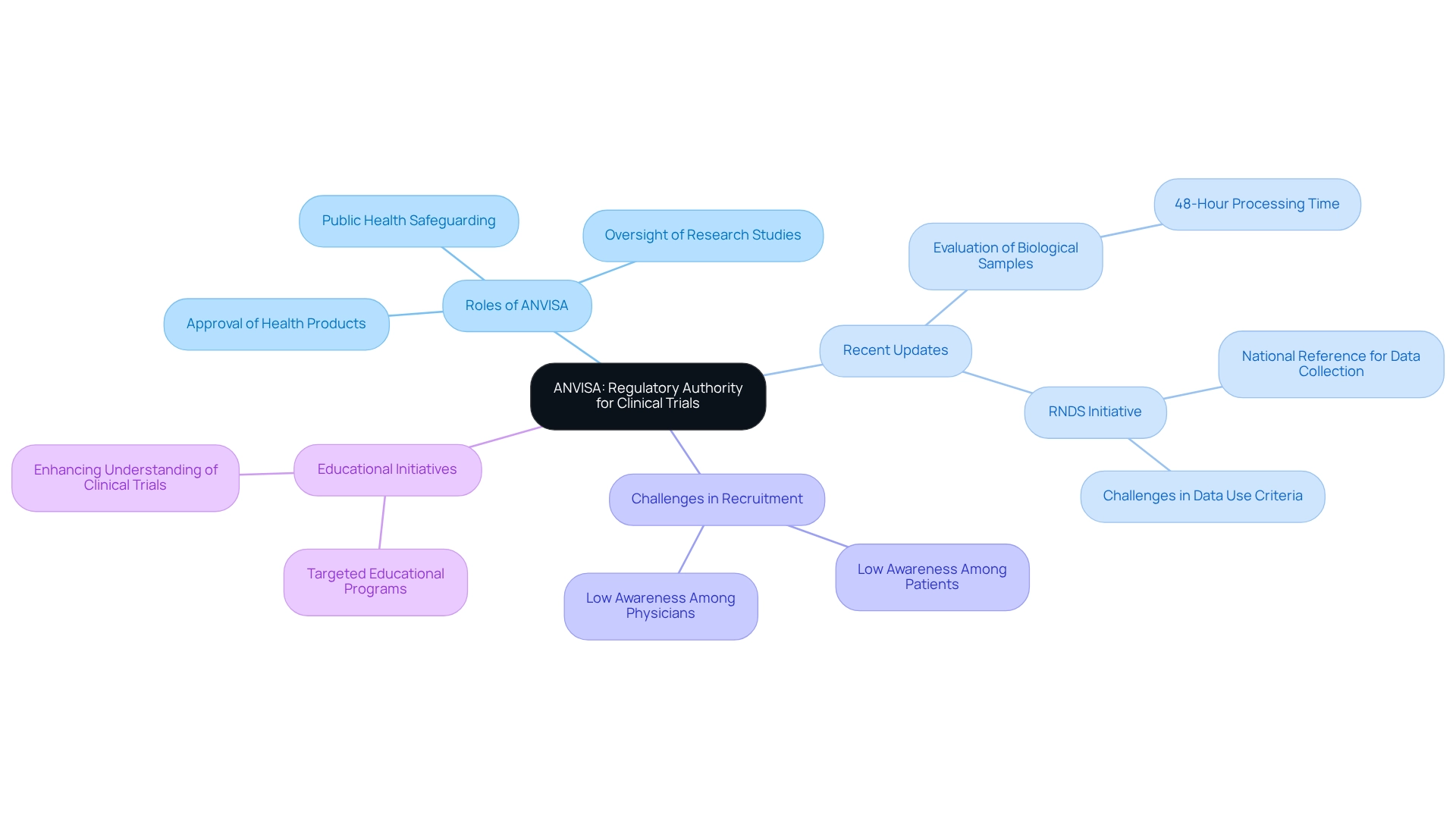
The Brazilian Health Regulatory Agency (ANVISA) is integral to the regulation and oversight of health products, including drugs and medical devices. Established to guarantee the safety and effectiveness of these products, ANVISA plays a crucial role in the approval and oversight of research studies throughout Brazil. Its regulations are meticulously crafted to safeguard public health while promoting the advancement of innovative medical solutions.
Recent updates indicate that ANVISA will evaluate and distribute human biological samples intended for research within 48 hours of their arrival in Brazil, provided all legal requirements are fulfilled. This accelerated process is expected to significantly enhance the efficiency of medical studies, particularly for multicenter research, where the coordinating center investigator must prepare and submit consolidated Serious Adverse Event (SAE) reports to the ethics committee.
The establishment of the RNDS (National Health Data Network) is anticipated to serve as a national reference for healthcare data collection. However, challenges persist in establishing data use criteria for compliance purposes, underscoring the significance of this initiative in enhancing approval rates for research studies and ensuring that data gathering aligns with compliance standards.
Despite these advancements, a survey revealed a concerning lack of awareness among both patients and doctors regarding the advantages of research, contributing to low recruitment levels for studies in Brazil. The findings indicate that focused educational programs are essential to enhance awareness and comprehension of research studies, potentially leading to higher participation rates among patients and increased involvement from doctors.
In conclusion, understanding how ANVISA impacts clinical trial design is vital for researchers and sponsors intending to conduct studies in Brazil, as it establishes benchmarks for compliance and ethical behavior in research. The agency's evolving role and policy updates are crucial for comprehending how ANVISA influences clinical trial design in the realm of medical research. With bioaccess®'s expertise in managing extensive research services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, researchers can navigate these regulations effectively.
Furthermore, bioaccess® is committed to addressing the challenges of low recruitment through educational initiatives and tailored support, ensuring successful outcomes and adherence to compliance standards.
Carrying out clinical studies in Brazil necessitates strict adherence to several key stipulations established by the regulatory agency, illustrating the significant impact of ANVISA on clinical trial design to ensure compliance and participant safety. These requirements include:
As we look ahead to 2025, the approval procedure for medical studies in Brazil continues to evolve, demonstrating how ANVISA impacts clinical trial design by emphasizing the importance of timely ethical assessments. Recent statistics indicate that a notable percentage of research study applications receive approval, further illustrating ANVISA's commitment to promoting research while upholding strict ethical standards. Jarbas Barbosa, the current head of the agency, has remarked that "the centralization of analysis by the CEP/Conep System needs to be evaluated and takes as a reference the establishment of the deadline for analysis response, akin to that set by the oversight body by RDC 9/2015."
This highlights ANVISA's ongoing improvements in the approval process.
Case studies exemplify the effectiveness of Brazil's ethical approval process. For instance, the endorsement and oversight of medical studies in Brazil illustrate how ANVISA impacts clinical trial design, as they involve thorough assessments by ethical and governing bodies, ensuring adherence to established standards. Despite some claims from researchers that the approval process may impede new studies, ongoing enhancements in Brazilian standards reflect ANVISA's commitment to refining procedures while maintaining high-quality ethical evaluations.
Moreover, entities such as bioaccess® have effectively conducted research for regulatory submission, offering guidance for clinical device development initiatives and further demonstrating the practical elements of compliance. Additionally, bioaccess® specializes in various types of studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), providing a customized approach to trial management to ensure that each study is tailored to meet specific regulatory and participant needs.

Bioequivalence studies are crucial in the approval process for generic medications in Brazil, as mandated by the regulatory agency. These studies aim to demonstrate that a generic product is therapeutically equivalent to its branded counterpart, ensuring both products exhibit similar pharmacokinetic profiles and therapeutic effects in the body. The following key aspects are essential for compliance with ANVISA's stringent guidelines:
At bioaccess, we offer comprehensive clinical trial management services that encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. Our expertise, especially through professionals like Katherine Ruiz, who focuses on compliance matters for medical devices and in vitro diagnostics in Colombia, ensures that our clients navigate the intricacies of ANVISA's guidelines effectively.
In 2025, the significance of bioequivalence studies in Brazil is anticipated to rise, driven by a growing demand for generic medications and the necessity for strict oversight. The U.S. FDA's endorsement of 917 Abbreviated New Drug Applications (ANDAs) in 2022 indicates a growing trend in compliance that is likely to influence Brazil's market as well. Additionally, the case study titled "Strategic Initiatives in Bioequivalence Studies" illustrates how major players in the market are focusing on inorganic growth strategies, such as mergers and partnerships, to enhance their presence.
As the market evolves, it will be essential for stakeholders to understand how ANVISA impacts clinical trial design and the intricacies of effective bioequivalence study designs to navigate the complexities of drug development in this region. Furthermore, the anticipated growth in the Asia Pacific region's generics market, projected to register a CAGR of 8.8%, underscores the global trend towards increased demand for bioequivalence studies, further contextualizing their importance in Brazil.
Navigating the oversight framework of health authorities presents various challenges for researchers in Brazil, particularly in the realm of medical studies. Key issues include:
By proactively addressing these challenges, researchers can enhance their likelihood of successfully navigating the regulatory environment, ultimately fostering the advancement of cutting-edge medical technologies in Brazil. With over 20 years of experience in Medtech, bioaccess® exemplifies how a leading organization effectively connects innovative Medtech companies with opportunities for conducting research in Latin America. bioaccess® offers a tailored approach to overseeing research studies, ensuring adaptability and adherence to regulatory guidelines while emphasizing data security and client confidence.
With expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, bioaccess® is committed to accelerating the research process and advancing the development of medical devices.
The regulatory body's guidelines underscore the significant influence of ANVISA on clinical trial design, playing a vital role in shaping the framework of medical studies in Brazil. This ensures that such studies maintain high standards of scientific integrity and ethical responsibility. At bioaccess®, we leverage our extensive experience of over 20 years in managing clinical studies to adeptly navigate these guidelines. Our comprehensive clinical study management services encompass feasibility assessments, site selection, compliance reviews, study setup, import permits, project management, and reporting. Our expertise spans various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
Key considerations include:
In addition to these foundational components, the agency's influence extends to integrating specific protections in studies involving at-risk groups, such as women of reproductive age or expectant mothers. This commitment reflects ethical research practices and participant protection. For instance, additional safeguards are mandated to ensure awareness of risks and impacts on fertility and pregnancy.
Moreover, ANVISA's enhanced analysis process allows for the inclusion of documentation from comparable foreign oversight bodies, streamlining the assessment of research study applications. This innovation improves the efficiency of the regulatory process, facilitating quicker access to new therapies while upholding rigorous safety and efficacy standards. As noted by Carmen Penido in her article 'Avaliação de ensaios clínicos no Brasil: histórico e atualidades,' grasping these guidelines is vital for navigating the research landscape in Brazil.
Furthermore, sponsors are required to ensure access to post-study medication supply programs for research participants, including those in early-terminated studies, underscoring their ethical obligations in medical research. Overall, understanding ANVISA's impact on clinical trial design is essential for compliance with the agency's guidelines, which guarantees the successful design and implementation of research studies in Brazil. Bioaccess® is committed to leading the way in Medtech research in Latin America, emphasizing innovation and regulatory excellence.
ANVISA enforces stringent statistical analysis requirements for clinical studies to uphold the integrity and validity of research findings. Key components of these requirements include:
The significance of following these statistical standards cannot be overstated, as they not only improve the credibility of the results but also illustrate how ANVISA impacts clinical trial design through strict regulatory oversight. As Negar Gharavi, Senior Director of Medical Writing & Regulatory Affairs, mentions, "BioPharma Services can assist in navigating your next drug development project from concept to clinic," emphasizing the essential role of compliance in successful research studies.
Additionally, researchers need to recognize the obligation to submit a yearly progress report within 60 days of the effective date of the investigational new drug, highlighting the significance of prompt reporting in research studies. This aligns with bioaccess's commitment to thorough trial management services, including reporting on study status and adverse events.
The successful certification of bioaccess by ANVISA exemplifies adherence to these standards, highlighting how ANVISA impacts clinical trial design to ensure that research conducted in Brazil meets the highest quality benchmarks. This certification process emphasizes how ANVISA impacts clinical trial design by underscoring the importance of adhering to statistical analysis criteria, which are vital for the approval of research studies, including the import permit and nationalization of investigational devices.
As Brazil progresses in its research study environment, particularly with the introduction of new regulations effective January 1, 2025, understanding how ANVISA impacts clinical trial design and incorporating these statistical requirements will be crucial for researchers managing the intricacies of studies in the region. Furthermore, Brazil's centralized registration procedure for research ethics committees (ECs) further influences the oversight framework, requiring institutional level EC approval for each research site, coordinated by the National Research Ethics Commission (CONEP).
Safety oversight is a crucial element of research studies, and the regulatory agency has established strict guidelines concerning adverse event documentation to guarantee participant safety and adherence to regulatory standards. At bioaccess®, we leverage over 20 years of expertise in managing medical device clinical trials, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), to ensure that our clients meet these critical requirements. Key components include:
Alongside these criteria, it is essential for researchers to recognize that research ethics committees cannot impose charges for evaluating medical study protocols. This highlights the dedication to ethical supervision in medical research. As highlighted by Josiany Carlos de Souza, "the risks are common to medical devices; therefore, it is crucial that there are preventative measures to avoid them, for example, training users to use the products, maintenance, improving quality, and reporting adverse events to manufacturers."
This proactive method not only complies with regulatory standards but also promotes a culture of safety and responsibility in research studies. With bioaccess®'s extensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, we are well-prepared to handle the complexities of research in Latin America.
Recent legislative changes in Brazil have profoundly influenced ANVISA's clinical trial regulations, with several key developments shaping the landscape:
The impact of Law No. 14.874/2024 illustrates how ANVISA influences clinical trial design. The research sector is increasingly recognizing this influence. For instance, Argentina has experienced an extraordinary 79% growth in research studies and a 130% increase in healthcare R&D funding from 2017 to 2021, driven by comparable government reforms.
This trend underscores Latin America's growing capacity as a hub for medical research, attracting international studies and funding in innovation. Furthermore, Mexico's COFEPRIS recently authorized the first Mexico-developed COVID-19 vaccine, highlighting the advancements in oversight frameworks across the region.
As Jucelia Maria Guedert noted, "It is essential that the new 'National System for Ethics in Research with Human Beings' considers social control, works in line with Anvisa and is adequately funded to fulfill its mandate." This statement underscores the significance of ethical considerations within the context of the new regulations. As Brazil continues to adapt its regulatory environment, understanding how ANVISA impacts clinical trial design holds significant implications, paving the way for more efficient and effective research practices, supported by Bioaccess®'s expertise in regulatory navigation and tailored solutions.
In conclusion, the evolving regulatory landscape for clinical trials in Brazil, propelled by ANVISA's unwavering commitment to safety, efficiency, and innovation, is crucial for the future of medical research. Recent legislative changes, including Law No. 14.874/2024, are designed to streamline approval processes, thereby reducing bureaucratic hurdles for researchers and enhancing the predictability of trial outcomes. This pivotal shift positions Brazil as a competitive hub for clinical research in Latin America, emphasizing the necessity for stakeholders to stay informed and engaged.
For researchers and sponsors aiming to conduct successful clinical trials, understanding ANVISA's requirements is essential. Compliance with comprehensive Clinical Trial Applications, Good Clinical Practices, and timely reporting of adverse events is paramount. Additionally, the implementation of bioequivalence studies and rigorous safety monitoring highlights the agency's dedication to public health standards while fostering an innovative environment.
As Brazil navigates the complexities of clinical trial regulations, collaboration with experienced partners, such as bioaccess®, becomes increasingly vital. This partnership can provide the necessary support to manage clinical trials efficiently, ensuring adherence to regulatory standards while addressing challenges like low recruitment rates. By prioritizing education and awareness initiatives, stakeholders can unlock the potential for increased participation in clinical research, ultimately benefiting public health and advancing medical science.
What is the role of ANVISA in Brazil?
The Brazilian Health Regulatory Agency (ANVISA) is responsible for the regulation and oversight of health products, including drugs and medical devices, ensuring their safety and effectiveness. It plays a crucial role in the approval and oversight of research studies throughout Brazil.
How has ANVISA improved the process for evaluating human biological samples for research?
ANVISA will evaluate and distribute human biological samples intended for research within 48 hours of their arrival in Brazil, provided all legal requirements are met. This accelerated process is designed to enhance the efficiency of medical studies, especially for multicenter research.
What is the RNDS and its significance?
The RNDS (National Health Data Network) is expected to serve as a national reference for healthcare data collection. It aims to improve approval rates for research studies and ensure that data gathering aligns with compliance standards, although challenges remain in establishing data use criteria.
What challenges does Brazil face regarding research study recruitment?
A survey indicated a lack of awareness among patients and doctors about the advantages of research, contributing to low recruitment levels for studies in Brazil. Focused educational programs are necessary to enhance awareness and comprehension of research studies.
What are the key requirements for conducting clinical studies in Brazil?
Key requirements include: 1. Submission of a Clinical Trial Application (CTA) with detailed study protocol and investigator qualifications. 2. Obtaining ethical approval from local ethics committees and, in some cases, the National Commission for Ethics in Research (CONEP). 3. Adherence to Good Clinical Practices (GCP) to ensure data integrity and participant safety. 4. Prompt reporting of adverse events to maintain participant safety and regulatory compliance.
How does ANVISA influence clinical trial design?
ANVISA establishes benchmarks for compliance and ethical behavior in research, impacting clinical trial design significantly. Understanding ANVISA's role is vital for researchers and sponsors conducting studies in Brazil.
What support does bioaccess® provide to researchers in navigating ANVISA regulations?
Bioaccess® offers expertise in managing research services, including assistance in preparing and submitting CTAs, navigating ethical requirements, ensuring GCP compliance, and managing adverse event reporting, all aimed at facilitating successful study outcomes.
What is the current state of the approval process for medical studies in Brazil?
The approval process for medical studies in Brazil is evolving, with a notable percentage of research study applications receiving approval. ANVISA is committed to promoting research while upholding strict ethical standards, and ongoing improvements are being made to the approval process.