


The article delineates four critical steps for harnessing market entry accelerators in Mexico's medical device sector. These steps emphasize the importance of:
Insights into the advantages of accelerators, such as access to local expertise and expedited regulatory approval processes, underscore their significance for companies aspiring to thrive in Mexico's rapidly expanding healthcare market.
Navigating the complexities of the Mexican medical device market presents both challenges and opportunities for businesses aiming to expand their reach. Market entry accelerators have emerged as vital resources, offering tailored support to help companies excel in this competitive landscape. By leveraging these programs, businesses can unlock essential insights and connections that drive success.
However, what criteria should they prioritize when selecting the right accelerator? This article delves into the critical steps for effectively engaging with market entry accelerators in Mexico, ensuring that companies can capitalize on the booming healthcare sector while adeptly navigating regulatory hurdles.
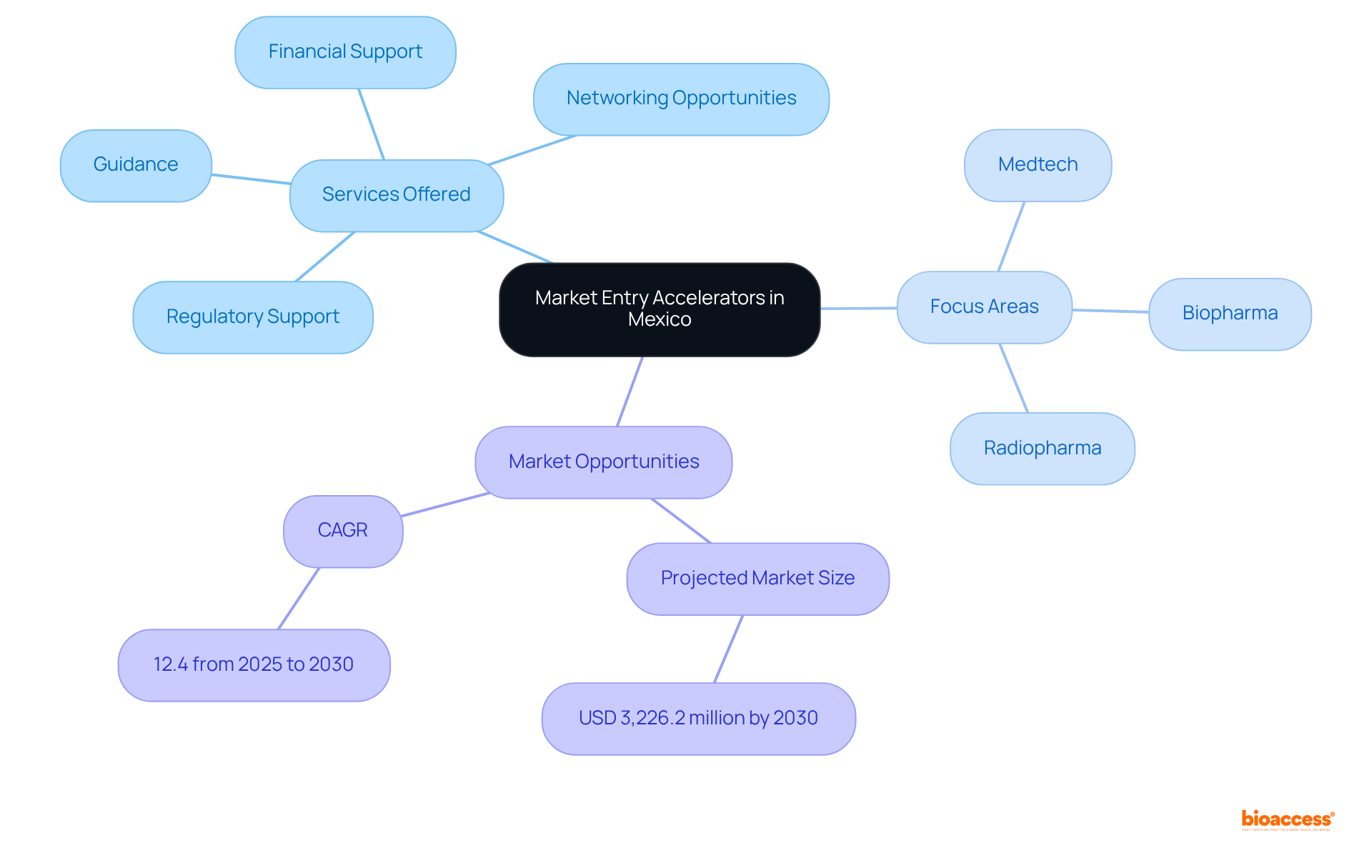
The market entry accelerator Mexico medical devices represents organized initiatives that empower startups and established businesses to navigate the complexities of the local medical device landscape. These programs offer essential resources, including guidance, financial support, and networking opportunities, which are vital for thriving in a competitive environment. They enhance understanding of local regulations, foster connections with potential partners, and aid in refining business strategies. By leveraging Mexico's unique healthcare ecosystem—characterized by regulatory flexibility and diverse patient demographics—companies can significantly boost their prospects for successful sector entry.
To capitalize on the advantages of these programs, businesses should familiarize themselves with the specific services available, such as access to local expertise, industry insights, and logistical support. Notably, bioaccess® provides comprehensive access solutions, including the 'Sprint reglamentario' for regulatory approval, achieving approvals in as little as 6-8 weeks—substantially faster than the typical 6-12 months required in the US/EU. Understanding the focus areas of each accelerator—whether they cater to Medtech, Biopharma, or Radiopharma—enables businesses to align their objectives with the most suitable program. This strategic alignment is essential for harnessing the full potential of the market entry accelerator Mexico medical devices, ultimately enhancing startup success in Mexico's burgeoning healthcare equipment sector.
The Mexico intelligent healthcare equipment sector is projected to reach USD 3,226.2 million by 2030, with a compound annual growth rate (CAGR) of 12.4% from 2025 to 2030, underscoring the significant opportunities available for firms entering this arena. With chronic conditions such as diabetes and heart disease being prevalent, the demand for advanced healthcare equipment is critical. By addressing these market needs, entities like bioaccess® play an indispensable role in fostering the development and commercialization of innovative healthcare technologies.
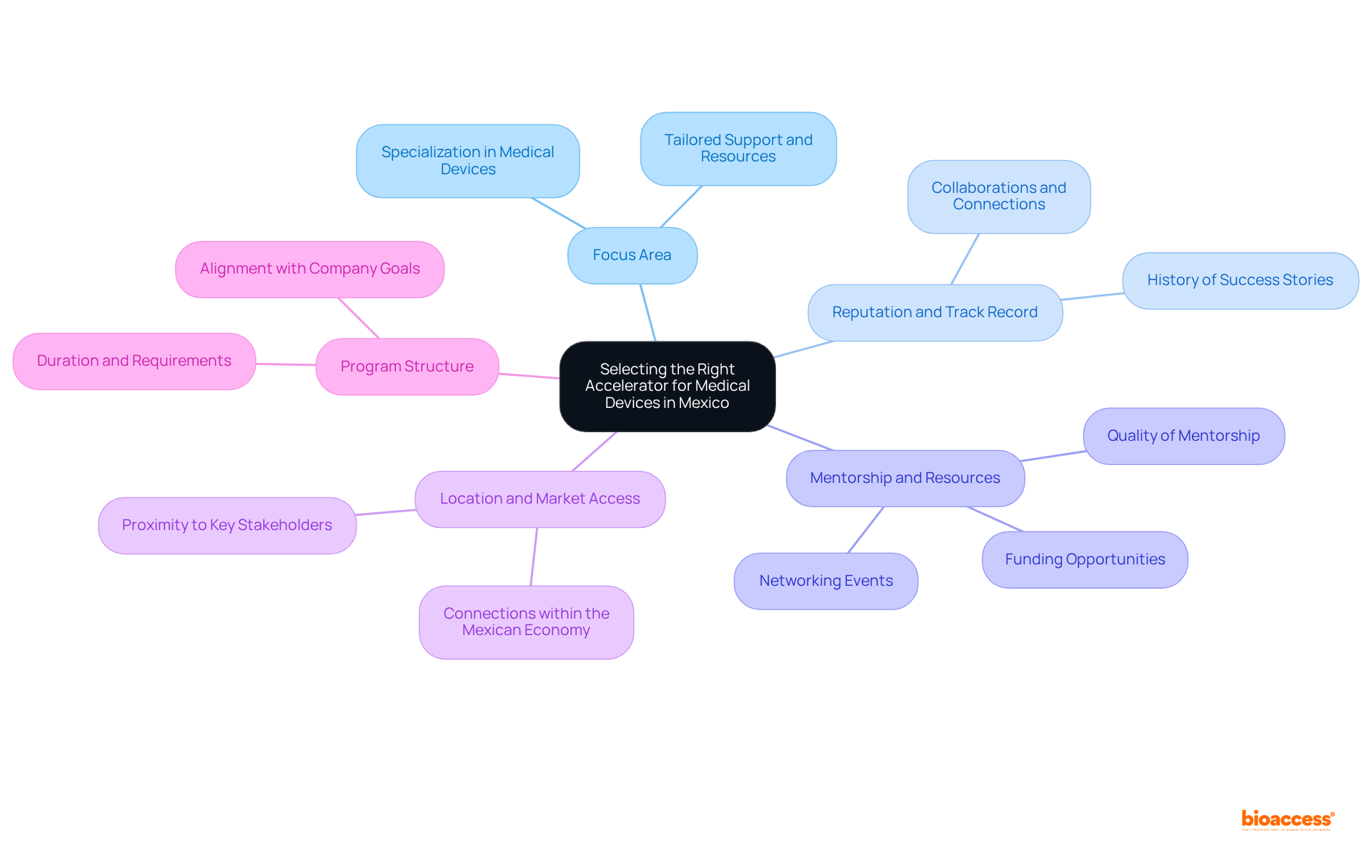
When selecting the right market entry accelerator Mexico medical devices, it is crucial for companies to consider several essential factors.
Focus Area: It is imperative to select a program that specializes in medical devices or related fields. This specialization ensures tailored support and resources that are directly relevant to your industry.
Reputation and Track Record: Examine the program's history, including success stories and collaborations. A trustworthy program with a proven track record can provide important connections and enhance your credibility within the industry.
Mentorship and Resources: Assess the quality of mentorship and resources available. Seek programs that connect you with industry specialists, funding opportunities, and networking events, which are vital for growth and development.
Location and Market Access: Evaluate the program's location and its connections within the Mexican economy. Proximity to key stakeholders, such as regulatory bodies and healthcare providers, can significantly bolster your market entry efforts.
Program Structure: Review the program's structure, duration, and requirements to ensure they align with your company's goals and timelines.
By thoroughly evaluating these factors, companies can select a program that serves as a market entry accelerator Mexico medical devices, ultimately enhancing their chances of successful entry into the burgeoning sector.
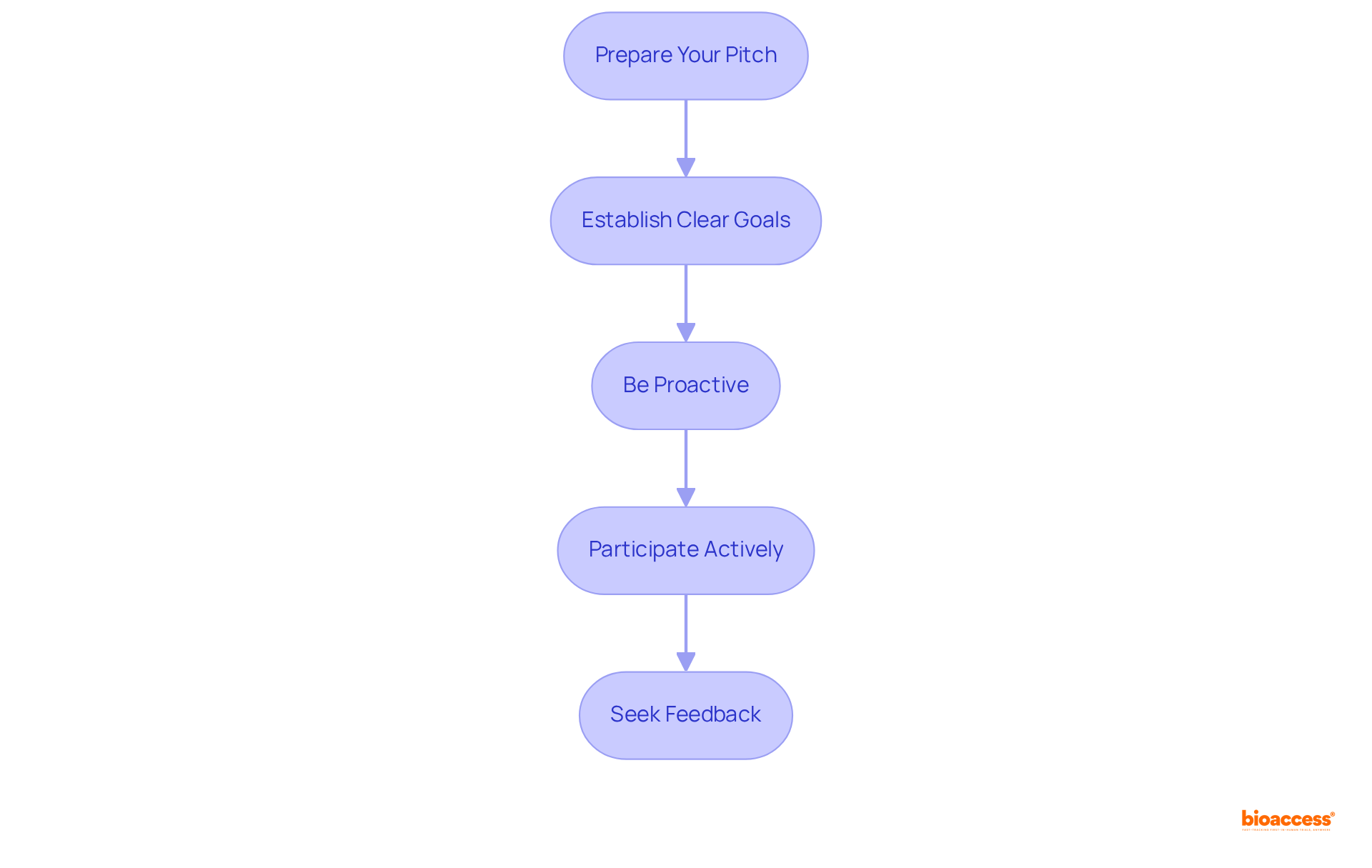
To engage effectively with an accelerator, it is crucial to follow these strategic steps:
By implementing these strategies, companies can foster a collaborative environment that maximizes the advantages of their accelerator experience.
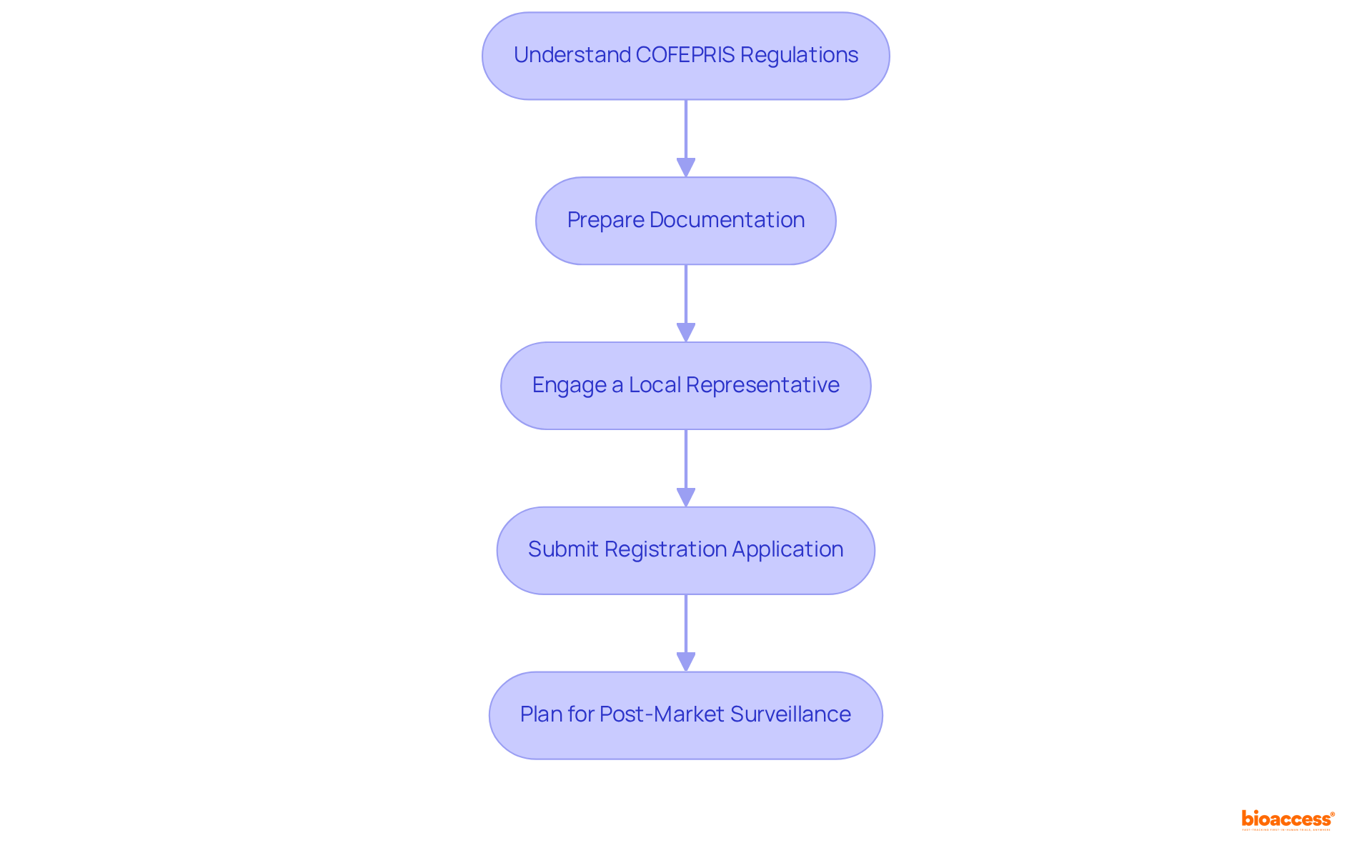
Navigating regulatory compliance and market access in Mexico requires a strategic approach, encompassing several essential steps:
Understand COFEPRIS Regulations: Familiarize yourself with the regulations set by COFEPRIS (Federal Commission for the Protection against Sanitary Risks), the primary authority supervising the registration of health-related equipment in Mexico. A thorough understanding of your equipment's classification is vital for ensuring compliance, as Class III items necessitate extensive clinical data and rigorous examination. bioaccess® specializes in managing Early-Feasibility Studies and Post-Market Clinical Follow-Up Studies, ensuring compliance with COFEPRIS regulations.
Prepare Documentation: Compile all necessary documentation for registration, including technical files, clinical data, and labeling requirements. It is crucial that all documents are in Spanish and adhere to COFEPRIS standards. The introduction of new Good Manufacturing Practices (GMP) guidelines, effective November 30, 2025, is expected to streamline this process, potentially reducing approval times for high-risk products by up to 30%. This statistic underscores the importance of staying informed about regulatory changes.
Engage a Local Representative: If your company lacks a physical presence in Mexico, appoint a local representative to manage the registration process and serve as a liaison with COFEPRIS. This step is essential for navigating the complexities of the regulatory landscape and ensuring timely communication. Engaging a local expert, such as bioaccess®, can provide invaluable insights into the regulatory environment and facilitate smoother interactions with COFEPRIS.
Submit Registration Application: Complete and submit your registration application to COFEPRIS, ensuring that all required documents are included. Be prepared for potential follow-up questions or requests for additional information, as the standard review process generally takes around 12 months, while third-party reviews can take 3 to 6 months. Furthermore, COFEPRIS charges for registration of healthcare equipment vary from $500 to $1,100 USD, depending on the category, which is a significant factor for budgeting.
Plan for Post-Market Surveillance: After your product receives approval, establish a robust plan for post-market surveillance to monitor its performance and ensure ongoing compliance with regulatory requirements. Effective post-market strategies, including vigilance systems for reporting adverse events, are critical for maintaining product safety and efficacy. Ongoing training and education for staff on adherence practices and regulatory updates are vital to ensure compliance and adapt to any changes in regulations.
By adhering to these steps, companies can successfully navigate the regulatory landscape and utilize the market entry accelerator Mexico medical devices to gain access to the burgeoning market, positioning themselves for success in the sector.
Leveraging a market entry accelerator in Mexico's medical device sector significantly enhances a company's chances of success. These programs provide essential resources and support, offering a strategic pathway to navigate the complexities of the local healthcare landscape. By aligning with the right accelerator, businesses can tap into valuable expertise, streamline regulatory processes, and ultimately position themselves for growth in a rapidly evolving market.
Key insights regarding the importance of selecting a suitable accelerator based on focus areas, reputation, and available resources have been shared throughout the article. Engaging effectively with these programs requires clear goal-setting, proactive networking, and active participation. Furthermore, understanding regulatory compliance and market access strategies is crucial for overcoming the challenges posed by COFEPRIS and ensuring a smooth entry into the Mexican market.
The potential for growth in Mexico’s intelligent healthcare equipment sector is substantial, driven by a rising demand for innovative medical devices. By taking advantage of market entry accelerators, companies can meet the needs of a diverse patient population and contribute to advancing healthcare technologies. Embracing this opportunity can lead to transformative outcomes, making it imperative for businesses to act strategically and engage with the right partners in this promising landscape.
What are market entry accelerators in Mexico for medical devices?
Market entry accelerators in Mexico for medical devices are organized initiatives that help startups and established businesses navigate the local medical device landscape by providing essential resources such as guidance, financial support, and networking opportunities.
How do these programs benefit businesses?
These programs enhance understanding of local regulations, foster connections with potential partners, and aid in refining business strategies, which are vital for thriving in a competitive environment.
What specific services do market entry accelerators offer?
They offer access to local expertise, industry insights, and logistical support, which help businesses capitalize on the advantages of the Mexican healthcare ecosystem.
How does bioaccess® assist companies in the market entry process?
Bioaccess® provides comprehensive access solutions, including the 'Sprint reglamentario' for regulatory approval, achieving approvals in as little as 6-8 weeks, significantly faster than the typical 6-12 months in the US/EU.
What should businesses consider when choosing an accelerator?
Businesses should understand the focus areas of each accelerator, whether they cater to Medtech, Biopharma, or Radiopharma, to align their objectives with the most suitable program.
What is the projected growth of the intelligent healthcare equipment sector in Mexico?
The intelligent healthcare equipment sector in Mexico is projected to reach USD 3,226.2 million by 2030, with a compound annual growth rate (CAGR) of 12.4% from 2025 to 2030.
Why is there a demand for advanced healthcare equipment in Mexico?
There is a critical demand for advanced healthcare equipment due to the prevalence of chronic conditions such as diabetes and heart disease, highlighting the opportunities for firms entering this market.