


Are you looking to expand your clinical research operations into one of the world's fastest-growing markets? Latin America's clinical trials sector is experiencing unprecedented growth, with a projected market value of $3.2 billion by 2025.
As the region emerges as a prime destination for clinical studies, choosing the right Latin America contract research organization (CRO) becomes crucial for your research success. These organizations offer comprehensive support across various therapeutic areas, from early feasibility studies to complex medical device clinical trials.
The landscape of contract research organizations in Latin America has evolved significantly, with several key players establishing robust infrastructures across Mexico, Brazil, Colombia, and other strategic locations. Each brings unique capabilities, specialized expertise, and extensive regional networks to the table.
To help you make an informed decision, we've analyzed seven leading CROs that are setting new standards in Latin American clinical research. Our comprehensive evaluation covers their service offerings, geographical reach, and specialized capabilities in managing both pharmaceutical and medical device clinical trials.
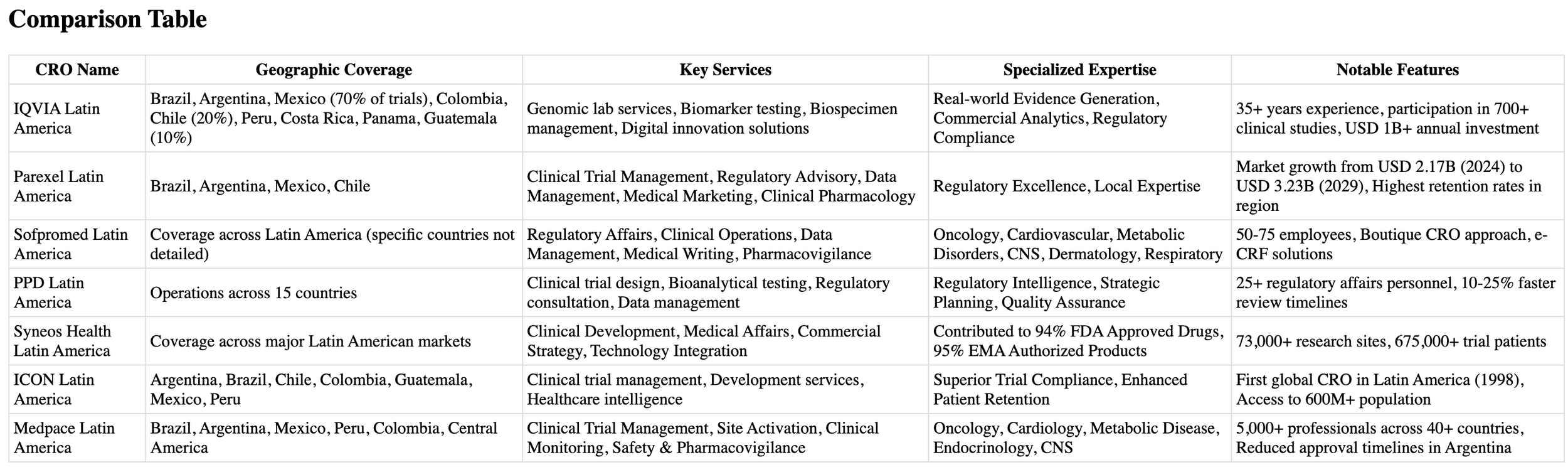
When seeking a partner for your clinical research initiatives in Latin America, IQVIA stands out as one of the region's most comprehensive laboratory services organizations. Their end-to-end solutions and enterprise-wide management capabilities make them a formidable presence in the clinical research landscape.
IQVIA combines cutting-edge technology with over 35 years of clinical vaccine development experience [1]. Their service portfolio includes:
The organization's commitment to innovation is evident through their dedicated Digital Innovation department, which focuses on creating agile solutions that enhance traceability, productivity, and efficiency [1].
Your research benefits from IQVIA's data-driven approach and specialized expertise across multiple domains. They leverage established relationships to identify optimal research sites and implement patient-centered recruitment strategies [2]. Their technical proficiency extends to:
IQVIA maintains a robust presence across key Latin American markets, with significant investments and infrastructure. In 2019, the organization, along with other industry leaders, contributed to approximately USD 980 million in clinical trials across Brazil, Argentina, and Mexico [4]. Their regional coverage includes:
The organization's commitment to the region is reflected in their participation in nearly 700 clinical studies, with investments exceeding USD 1 billion in a single year [5]. Their presence in major urban centers, where over 80% of Latin America's population resides, ensures efficient patient recruitment and retention [4].
Partnering with a well-established contract research organization can significantly accelerate your clinical research initiatives in Latin America. Parexel, with its extensive regional experience since 2002, offers you comprehensive solutions across multiple therapeutic areas.
Your clinical research benefits from Parexel's full spectrum of Phase I to IV clinical development services, supported by over 21,000 global professionals [6]. The organization excels in:
The organization's commitment to Latin America is evidenced by impressive market growth projections, with the regional clinical trials market expected to expand from USD 2.17 billion in 2024 to USD 3.23 billion by 2029 [7]. Your research benefits from:
Your research gains access to strategically positioned facilities across key markets. Parexel maintains four primary locations in Latin America:
The organization's infrastructure is further strengthened through partnerships with local CROs and collaboration with ABRACRO (Brazilian Association of Clinical Research Representative Organizations) [7]. This network enables you to leverage:
Parexel's commitment to the region is demonstrated through continuous infrastructure expansion and strategic partnerships, ensuring your clinical research benefits from both global expertise and local insights.
For efficient and cost-effective clinical research management in Latin America, Sofpromed offers a streamlined approach with its boutique CRO services. As a smaller organization with 50-75 employees, your research benefits from personalized attention while maintaining high-quality standards [11].
Your clinical trials gain from Sofpromed's comprehensive service portfolio that spans the entire research lifecycle. Their operations encompass:
The organization's commitment to operational excellence is enhanced through innovative e-CRF solutions and specialized web tools for managing medical images and biological samples [13].
Your research benefits from Sofpromed's expertise across multiple therapeutic areas, with particular strength in:
Therapeutic AreaSpecialized CapabilitiesOncologyPhase I-IV trialsCardiovascularFull-service managementMetabolic DisordersComprehensive monitoringCentral Nervous SystemData analysis expertiseDermatologyPatient recruitmentRespiratory DiseasesSafety monitoring [14]
The organization's biometrics team consists of highly qualified professionals ensuring accuracy and efficiency in data processing [15].
Your clinical research gains access to Sofpromed's extensive network spanning multiple regions. Their strategic presence includes:
The organization leverages its global presence to provide strategic guidance on country selection for cost optimization while maintaining quality standards [11]. Their approach to site networks enhances trial efficiency through:
Transform your clinical research initiatives with PPD's extensive Latin American network, backed by more than 25 dedicated regulatory affairs personnel supporting operations across 15 countries [17].
Your research benefits from PPD's comprehensive end-to-end solutions, including:
The organization's commitment to operational excellence is evident through their innovative approach to regulatory submissions, which has resulted in 10-25% faster review timelines across the region [19].
Your clinical studies gain from PPD's robust understanding of diverse local regulations and strong relationships with regulatory authorities [17]. Their expertise is demonstrated through:
The organization's regulatory team maintains trust-based relationships with individual authorities, ensuring efficient navigation of country-specific requirements while maintaining the highest safety standards [19].
Your research leverages PPD's advanced digital infrastructure and strategic regional presence. Their technological capabilities include:
The organization's regional coverage spans major Latin American markets, with state-of-the-art facilities and experienced researchers particularly concentrated in major cities [20]. Their infrastructure supports:
PPD's commitment to digitalization has significantly enhanced trial efficiency, with complete trial packages submission strategy reducing regulatory approval times [20]. Their infrastructure continues to evolve, incorporating new technologies and platforms to support increasingly complex clinical trials [18].
Leverage the power of integrated biopharmaceutical solutions with Syneos Health, a dynamic force in Latin American clinical research. Their unique approach combines clinical, medical affairs, and commercial insights to accelerate your path to market success.
Your clinical research benefits from Syneos Health's comprehensive integration of services. Their solutions encompass:
Their innovative approach places both you and your patients at the center, streamlining processes while maintaining the highest quality standards [21].
Your research gains credibility through Syneos Health's impressive track record. Over the past five years, they have:
Their expertise extends across multiple therapeutic areas, with a proven ability to navigate complex regulatory landscapes while maintaining consistent quality standards [22].
Your clinical trials benefit from Syneos Health's extensive regional presence, supported by their global network of 29,000 employees across 110 countries [21]. Their infrastructure includes:
The organization's commitment to Latin America is evidenced through their comprehensive support system, which includes:
Their patient-centric approach ensures high retention rates while their technological infrastructure supports efficient trial management across the region. The organization's commitment to diversity and inclusion creates an environment where innovative solutions thrive, directly benefiting your research outcomes [21].
Discover why ICON, the pioneer of clinical research in Latin America, continues to lead the region's transformation in clinical trials. As the first global CRO to establish operations in Latin America in 1998, ICON brings unparalleled experience to your research initiatives [24].
Your clinical trials benefit from ICON's comprehensive range of services, delivered through a network of over 41,160 professionals across 108 locations globally [25]. Their capabilities extend across:
The organization's commitment to quality has earned them prestigious recognition, including the PharmaTimes Clinical Research Company of the Year award in 2021 [25].
Your research gains from ICON's deep understanding of local regulations and cultural nuances. Their expertise is demonstrated through:
Your clinical studies leverage ICON's strategic presence across six key markets [24]:
The organization's regional infrastructure supports your research through:
ICON's commitment to advancing healthcare innovation is evident through their continuous investment in technology and infrastructure. Their presence in major metropolitan areas ensures access to concentrated patient populations, particularly beneficial for studies in cardiovascular diseases, arthritis, cancer, and infectious diseases, which show prevalence rates comparable to the United States [24].
Image Source: Medpace
Accelerate your clinical research initiatives with Medpace's strategic presence across Latin America, where regulatory expertise meets operational excellence. Their commitment to advancing safe and effective medical therapeutics sets them apart in the region's evolving research landscape.
Your clinical trials benefit from Medpace's comprehensive service portfolio, delivered by over 5,000 professionals across 40+ countries [27]. Their core services include:
The organization leverages local regulatory and therapeutic expertise across all major areas, ensuring your research meets the highest quality standards while maintaining efficiency [27].
Your research gains from Medpace's specialized expertise across multiple therapeutic domains:
Their commitment to excellence is particularly evident in Argentina, where new regulations have reduced clinical trial approval timelines from 160 to 70 business days or less [29]. This regulatory efficiency enables:
Your clinical studies leverage Medpace's extensive regional infrastructure, with strategic locations including:
The organization's regional presence offers unique advantages for your research:
Brazil's office expansion demonstrates Medpace's commitment to growth, with enhanced capabilities in Clinical Trial Management, Site Activation & Maintenance, Clinical Monitoring, and Safety & Pharmacovigilance [28]. This strategic growth enables improved quality, strategic thinking, and efficient delivery of services across the region [28].
Latin America stands as a prime destination for clinical research, backed by a projected market value of $3.2 billion by 2025. Each profiled CRO brings distinct advantages - from IQVIA's comprehensive laboratory services to Parexel's extensive regional experience, and from Syneos Health's integrated solutions to ICON's pioneering presence.
These organizations excel through their robust infrastructure spanning major markets like Brazil, Mexico, and Argentina. Their specialized expertise across therapeutic areas, combined with deep understanding of local regulations, helps streamline your clinical trials while maintaining the highest quality standards.
Patient recruitment and retention rates surpass global averages, thanks to concentrated urban populations and strong doctor-patient relationships. Advanced technological capabilities and standardized procedures ensure efficient trial management, data accuracy, and regulatory compliance.
Ready to elevate your medical device clinical trials in Latin America? Contact bioaccess® today to discover how our expertise can support your research initiatives!
Your choice of CRO partner significantly impacts research success. Consider factors like therapeutic expertise, regional coverage, and regulatory experience while selecting the organization best suited for your specific needs. These leading CROs demonstrate Latin America's capability to deliver world-class clinical research services while offering cost-effective solutions and access to diverse patient populations.
[1] - https://labs.iqvia.com/investigator-site-support-south-america-contact
[2] - https://www.iqvia.com/solutions/therapeutics/cell-and-gene-therapy
[3] - https://www.iqvia.com/events/2024/09/exploring-real-world-data-in-brazil-and-latin-america
[4] - https://www.clinicalleader.com/doc/latin-america-a-compelling-region-to-conduct-your-clinical-trials-0001
[5] - https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/valuing-the-research-based-pharmaceutical-industry-in-latin-america
[6] - https://www.parexel.com/
[7] - https://www.parexel.com/insights/article/latam-a-model-for-fsp-success
[8] - https://www.outsourcing-pharma.com/Article/2007/07/12/parexel-talks-clinical-research-in-latin-america/
[9] - https://www.biospace.com/parexel-international-strengthens-capabilities-in-latin-america-with-new-location-in-mexico-city
[10] - https://www.fiercebiotech.com/cro/parexel-expands-regional-distribution-center-and-supply-depot-network-to-enhance-global
[11] - https://www.sofpromed.com/affordable-cro-for-low-cost-clinical-trials-in-the-us-and-abroad
[12] - https://www.sofpromed.com/full-service-cro-worldwide-clinical-trials
[13] - https://www.linkedin.com/company/sofpromed
[14] - https://www.sofpromed.com/company
[15] - https://www.sofpromed.com/clinical-data-managers-for-clinical-trials
[16] - https://www.sofpromed.com/how-clinical-site-networks-enhance-clinical-trials
[17] - https://www.ppd.com/our-solutions/clinical/phase-ii-iv-clinical-trial-management/regulatory-affairs/global-regulatory-affairs/regulatory-development/
[18] - https://www.ppd.com/blog/growing-role-of-contract-research-organizations-in-clinical-trials/
[19] - https://www.ppd.com/wp-content/uploads/2020/01/2019-Oct-Regulatory-Rapporteur-Latin-America-Regulatory.pdf
[20] - https://mexicobusiness.news/health/news/latam-offers-great-potential-clinical-studies-rd-destination
[21] - https://www.syneoshealth.com/careers/jobs/15185014-sr-site-activation-manager-sponsor-dedicated-home-based-mexico
[22] - https://www.syneoshealth.com/clinical-corporate-careers/jobs/15182378-sr-dir-ssu-and-regulatory
[23] - https://www.intercash.com/syneos-health-case-study
[24] - https://www.iconplc.com/about/icon-in-latin-america
[25] - https://en.wikipedia.org/wiki/ICON_PLC
[26] - https://www.linkedin.com/company/icon-plc-2
[27] - https://careers.medpace.com/jobs/10162?lang=en-us
[28] - https://www.medpace.com/careers/blog/medpace-brazil-office-updates/
[29] - https://www.medpace.com/blog/clinical-research-in-argentina/
[30] - https://www.medpace.com/blog/spotlight-on-mexico/