


The article centers on the ANVISA guidelines for medical device trials, underscoring their essential role in ensuring patient safety, data integrity, and regulatory compliance in Brazil. It highlights recent updates to these guidelines, including new classification rules and streamlined processes. These enhancements not only improve the efficiency of clinical trials but also foster innovation within the healthcare sector, thereby safeguarding public health.
In Brazil, the regulatory landscape for medical devices is significantly shaped by ANVISA, the Brazilian Health Regulatory Agency, a pivotal entity in health product regulation since its establishment in 1999. With a mission to safeguard public health, ANVISA ensures that medical devices adhere to stringent safety, efficacy, and quality standards, thereby facilitating innovation while prioritizing patient safety.
Recent updates to its regulatory framework, which include new classification rules and streamlined approval processes, reflect an ongoing commitment to enhancing healthcare outcomes. As the agency adapts to the evolving medical technology sector, it becomes crucial for stakeholders to understand ANVISA’s guidelines in order to navigate the complexities of compliance and drive advancements in Brazil's healthcare system.
The Brazilian Health Regulatory Agency (Agencia Nacional de Vigilância Sanitária) has been at the forefront of health product regulation since its establishment in 1999. Its main objective is to safeguard and advance public health by ensuring the safety, effectiveness, and quality of health products, including healthcare instruments, pharmaceuticals, and food safety. The regulatory framework of the health agency, particularly the ANVISA guidelines for medical device trials, is essential for the approval process of healthcare instruments, requiring adherence to both national and international standards.
This framework not only supports innovation within the health technology sector but also emphasizes patient safety, rendering the agency an essential entity in Brazil's healthcare environment. As we look to 2025, the agency continues to adjust its regulations to improve public health outcomes. Recent updates, such as RDC 751/2022 and RDC 830/2023, have introduced new classification rules for healthcare products and streamlined various reporting and registration processes. These changes aim to replace older regulations, thereby improving clarity and efficiency for companies navigating the registration landscape in Brazil.
ANVISA's dual approval routes—notification for Class I and II products and complete approval for Class III and IV products—reflect its commitment to a structured regulatory environment that supports both innovation and safety. The impact of ANVISA's regulations on public health in Brazil is significant. By ensuring stringent supervision of healthcare instruments, the agency plays a crucial role in protecting patient safety and improving the effectiveness of healthcare solutions. Continuous Post-Marketing Surveillance is mandated to monitor performance, ensuring ongoing safety and effectiveness after approval.
This proactive method is crucial for sustaining public confidence in health technologies. Furthermore, the role of the agency is reflected by analogous oversight bodies throughout Latin America, which encounter similar difficulties in balancing innovation with safety. Specialist viewpoints emphasize that Brazil's strict regulations, particularly the ANVISA guidelines for medical device trials, not only safeguard public health but also encourage a competitive landscape for trial assessments, leading to favorable results in clinical research.
As the terrain of healthcare technology changes, ANVISA remains a cornerstone of oversight excellence, ensuring that Brazil's healthcare system continues to flourish. Furthermore, the agency highlights that electromedical equipment must obtain INMETRO certification, and items with telecommunications features necessitate ANATEL certification. This extensive oversight strategy is additionally backed by financial contributions to telemedicine solutions, such as the US$3 million declared in 2022, which highlights the dedication to innovation in the health technology sector.
In this context, bioaccess® stands out as a prominent contract research organization (CRO) in Latin America, specializing in expedited clinical study services for healthcare products. With over 20 years of experience, bioaccess® offers comprehensive clinical trial management services, including feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and reporting. The expertise of bioaccess® in navigating the regulatory framework, particularly the ANVISA guidelines for medical device trials, ensures that Medtech startups can efficiently progress through the clinical trial phases, from early feasibility studies to pivotal studies and post-market follow-up.
Furthermore, bioaccess® is committed to ensuring information security and client trust, with established grievance and data protection procedures that address client concerns with compliance and transparency.

The ANVISA guidelines for medical device trials are essential in ensuring ethical conduct and scientific rigor. These regulations encompass several crucial components, including ethical approval, informed consent, and adherence to Good Clinical Practices (GCP). Key regulations include:
In Brazil, the ethical approval rates for medical device trials have shown improvement, with recent statistics revealing that compliance has achieved around 75%, reflecting a rising dedication to regulatory standards. For example, compliance with the Good Clinical Practices set forth by the health regulatory agency has reached commendable levels, promoting a stronger clinical research environment. Furthermore, case studies, such as the execution of the telehealth program by the Ministry of Health, demonstrate the practical application of these guidelines. This program, aimed at improving access to care, has successfully incorporated ethical standards, although it also highlights persistent challenges in ensuring coordinated care, particularly within the private sector. Moreover, research involving native communities must adhere to ethical standards, safeguarding the welfare and cultural integrity of participants, which is a vital element of ethical practices in clinical trials.
As the landscape of health technology trials evolves, staying updated on the latest ANVISA guidelines for medical device trials and compliance standards is crucial for Medtech companies seeking to navigate the oversight landscape successfully. Additionally, bioaccess® offers comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF). Our expertise ensures that clients can effectively manage the complexities of clinical trials while adhering to regulatory guidelines.
Furthermore, bioaccess® is committed to data protection and has established grievance procedures to address client concerns, enhancing transparency and trust in our services. The study funded by FastGrants and the Rainwater Charitable Foundation, registered under ClinicalTrials.gov number NCT04727424, exemplifies the importance of funding and oversight in maintaining compliance and ethical standards in clinical trials.
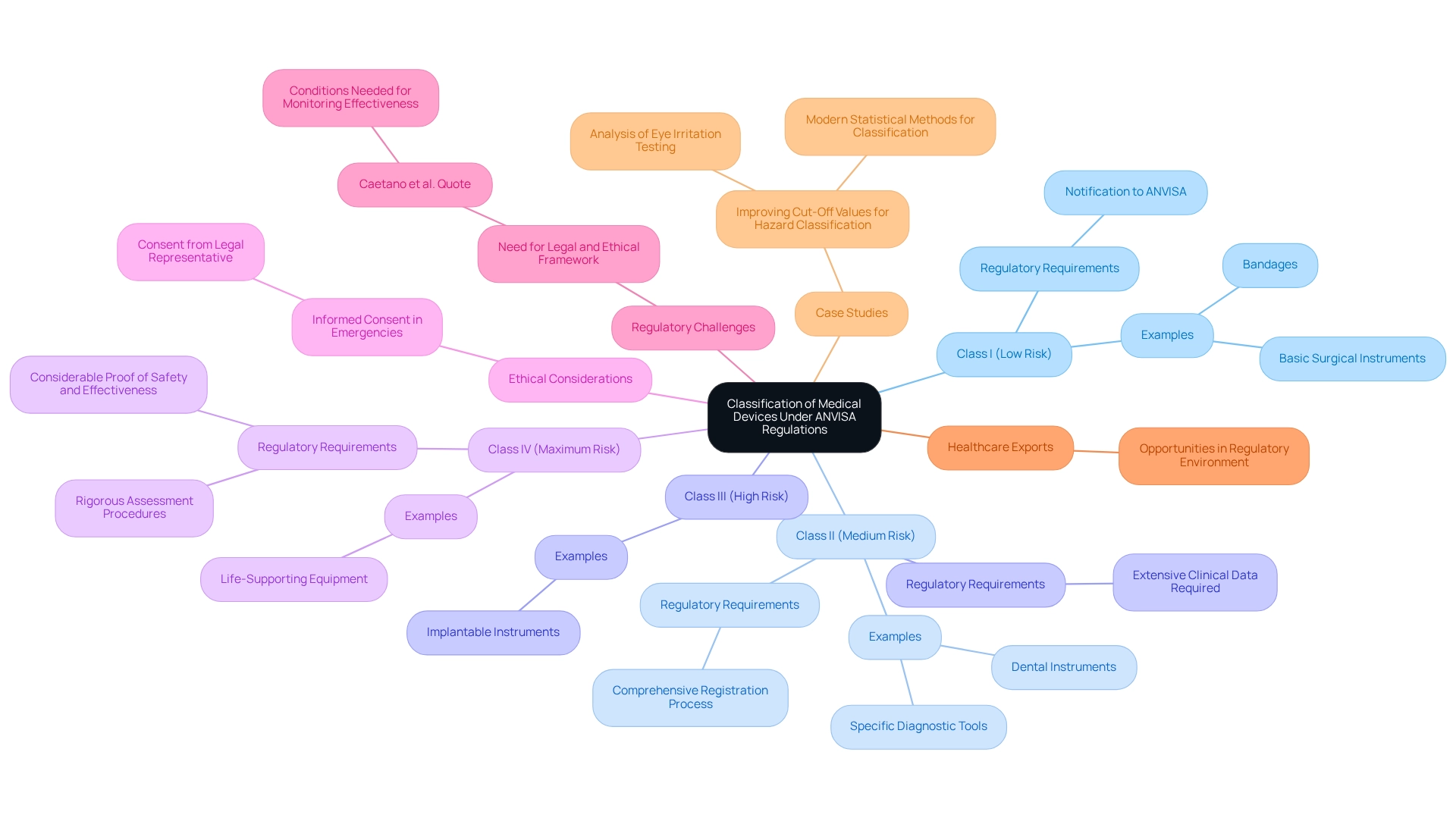
The health regulatory agency categorizes medical products into four unique groups according to their risk to patients, a crucial step in defining the regulatory course and ensuring adherence to established guidelines:
This classification system not only aids in compliance with regulations but also increases transparency in the healthcare product market. Recent trends indicate a growing emphasis on standardized and comparable information to enhance the overall safety profile of healthcare devices in Brazil. As highlighted in recent analyses, there is a pressing need for robust, standardized, and comparable information to enhance transparency in the pharmaceutical market.
In emergencies, if the signed informed consent form (ICF) cannot be obtained, the consent of the legal representative or guardian should be acquired, underscoring the importance of ethical considerations in the oversight framework surrounding healthcare devices.
Furthermore, a critical perspective from oversight experts, such as Caetano et al., emphasizes that a legal and ethical framework and structural conditions are needed to monitor effectiveness, conditions that Brazil still does not have. This highlights the ongoing challenges within the oversight environment.
The classification system is also pertinent in the context of healthcare exports, as opportunities in this area are influenced by the compliance landscape in Brazil. In 2025, ANVISA continues to enhance its classification procedures, reflecting ongoing advancements in healthcare technology and compliance practices. Comprehending these categories and their ramifications is essential for guaranteeing that health products fulfill the required safety criteria while promoting innovation in the healthcare field.
Additionally, bioaccess® offers comprehensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, and Post-Market Clinical Follow-Up Studies, ensuring compliance with INVIMA regulations in Colombia. A recent case study on improving cut-off values for hazard classification illustrates the importance of accurate classification methods and their implications for safety standards, further emphasizing the need for continuous improvement in regulatory practices.
The registration process for medical devices with ANVISA encompasses several critical steps that manufacturers must navigate to ensure compliance and successful market entry.
Pre-Submission Preparation: Manufacturers are required to compile a comprehensive technical file. This file must include essential components such as clinical data, risk assessments, and evidence of compliance with Good Manufacturing Practices (GMP). Notably, GMP certificates must be renewed every two years, emphasizing the importance of maintaining up-to-date documentation to ensure ongoing compliance.
Submission of Application: The application, along with the technical file, is presented to the regulatory agency for review. This submission must provide a detailed description of the medical device, its intended use, and the manufacturing processes involved. It is crucial to ensure that all information is accurate and complete to facilitate a smooth review process. Furthermore, producers must provide evidence of market approval in the country of origin for registration, although there are provisions for companies that cannot fulfill this requirement.
Regulatory Agency Review: The agency conducts a comprehensive assessment of the submitted documentation. This review may involve requests for additional information or clarification, which can extend the timeline for approval. Understanding the common challenges faced during this phase can help manufacturers prepare adequately, especially in relation to the ANVISA guidelines for medical device trials within the Latin American Medtech landscape.
Approval and Registration: Upon successful review, ANVISA issues a registration certificate, allowing the product to be marketed in Brazil. This registration is valid for a specified period, after which renewal is necessary to maintain compliance.
Recent updates to Brazilian healthcare equipment regulations, particularly the introduction of RDC 751/2022, have streamlined the registration process. This regulation simplifies the rules for categorizing healthcare instruments and simplifies the procedures for reporting, registering, modifying, renewing, and removing registrations. As noted in a statement by Margret Seidenfaden, "The Johner Institute team can support you with the approval of your medical devices in Brazil," highlighting the importance of expert guidance in navigating these changes.
Such policy updates reflect a more organized framework, ultimately aiming to enhance the efficiency of the approval process.
Navigating this registration process effectively is crucial for timely market entry and adherence to the ANVISA guidelines for medical device trials. Comprehending the typical duration required for health product registration with the regulatory agency and the success ratios of submissions can further guide strategic planning for producers looking to enter the Brazilian market. For those seeking assistance, bioaccess® provides extensive clinical trial management services, including Early-Feasibility Studies, First-In-Human Studies, and more, ensuring that your company is well-prepared to navigate the complexities of the registration process.
Recent updates to regulatory guidelines significantly affect the landscape of clinical trials and compliance. Key developments include:
These updates are not merely procedural; they represent a paradigm shift in how medical devices are regulated in Brazil under the ANVISA guidelines for medical device trials. Stakeholders, such as manufacturers and clinical research organizations, must adjust to these changes to ensure compliance and take advantage of the opportunities offered by a more organized oversight environment. For instance, bioaccess® specializes in managing various studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), leveraging over 20 years of experience in Medtech to ensure that clients navigate these regulatory updates effectively.
Additionally, as Silvio Guidi notes, "The firm represents companies throughout product development and marketing, including all pre-market clearance requirements for product safety, labeling, advertising, refurbishing, donation, and other marketing promotions." This highlights the critical nature of compliance with the new regulations on product development and marketing.
As the landscape evolves, the ability to navigate these updates will be essential for success in the Medtech sector. Furthermore, bioaccess® is committed to ensuring information security and client trust, addressing any concerns through established grievance and data protection procedures, thereby reinforcing its dedication to compliance and transparency.

Compliance with ANVISA guidelines presents significant challenges for medical device trials, categorized as follows:
To navigate these challenges effectively, companies should adopt proactive planning strategies, foster open communication channels, and cultivate a deep understanding of the compliance landscape. Implementing automated regulatory management systems has shown advantages, as demonstrated in case studies where companies optimized their submission processes, reducing delays and improving adherence to regulatory requirements. This strategic approach not only shortens approval timelines but also positions companies to capitalize on commercial opportunities more swiftly, ultimately driving innovation in the competitive Brazilian market.
Ensuring that healthcare instruments meet acceptable standards of quality, safety, and efficacy is crucial, as it directly influences the success of clinical trials and the overall progress of health technology.
To ensure compliance with ANVISA guidelines during medical device trials, it is essential to adopt the following best practices:
By adhering to these best practices and engaging with bioaccess®, researchers can significantly enhance their compliance efforts with the ANVISA guidelines for medical device trials and improve the overall quality of their clinical trials. Organizations that have successfully collaborated with the regulatory agency, such as bioaccess®, often report reduced timelines and smoother processes, underscoring the importance of thorough preparation and proactive communication.

Regulatory guidelines are essential for the effective execution of medical device trials in Brazil, ensuring several key outcomes:
In conclusion, a thorough understanding and compliance with the ANVISA guidelines for medical device trials are vital for advancing medical technology and enhancing patient outcomes in Brazil. The commitment to these regulations not only protects participants but also strengthens the overall integrity of clinical research. With over 20 years of experience in Medtech, bioaccess® is well-equipped to navigate these complexities effectively, leading the way in Medtech clinical research in Latin America.
ANVISA's role as Brazil's regulatory authority for medical devices is pivotal in ensuring public health and safety. The agency's comprehensive guidelines and recent updates reflect a commitment to fostering innovation while maintaining stringent safety and efficacy standards. By classifying medical devices into distinct categories, ANVISA streamlines the approval process, allowing for a more efficient market entry while safeguarding patient interests.
The challenges that manufacturers face—such as complex documentation requirements and lengthy approval processes—underscore the importance of thorough preparation and a proactive approach to compliance. Engaging with ANVISA early and implementing robust quality management systems can significantly enhance the likelihood of successful submissions.
Moreover, adherence to ANVISA guidelines not only protects trial participants but also enhances data integrity, ultimately supporting the advancement of medical technology. By bridging the gap between regulatory compliance and innovative research, stakeholders can build confidence among investors, healthcare professionals, and patients alike.
In this evolving landscape, organizations like bioaccess® play a crucial role in guiding Medtech companies through the complexities of the regulatory environment. Their expertise in clinical trial management ensures that compliance is met while fostering an atmosphere where innovation can thrive. As Brazil continues to refine its regulatory framework, understanding and leveraging ANVISA's guidelines will be essential for driving advancements in healthcare and improving patient outcomes.
What is the main objective of the Brazilian Health Regulatory Agency (ANVISA)?
The main objective of ANVISA is to safeguard and advance public health by ensuring the safety, effectiveness, and quality of health products, including healthcare instruments, pharmaceuticals, and food safety.
When was ANVISA established and what is its significance in Brazil's healthcare environment?
ANVISA was established in 1999 and is significant in Brazil's healthcare environment as it supports innovation in the health technology sector while emphasizing patient safety.
What are the recent updates to ANVISA's regulations?
Recent updates include RDC 751/2022 and RDC 830/2023, which introduce new classification rules for healthcare products and streamline reporting and registration processes.
What are the dual approval routes provided by ANVISA for healthcare products?
ANVISA offers notification for Class I and II products and complete approval for Class III and IV products.
How does ANVISA ensure ongoing safety and effectiveness of healthcare products after approval?
ANVISA mandates Continuous Post-Marketing Surveillance to monitor the performance of healthcare products, ensuring ongoing safety and effectiveness.
What role does bioaccess® play in the context of ANVISA and clinical trials?
Bioaccess® is a prominent contract research organization (CRO) in Latin America that specializes in expedited clinical study services for healthcare products, helping Medtech startups navigate the ANVISA guidelines efficiently.
What key regulations are included in the ANVISA guidelines for medical device trials?
Key regulations include: 1. Resolution RDC 10/2015, which establishes requirements for clinical trials involving health-related instruments. 2. Resolution RDC 751/2022, which classifies healthcare instruments by risk levels. 3. Resolution RDC 945/2024, which introduces enhanced procedures for clinical trial applications.
What improvement has been observed in ethical approval rates for medical device trials in Brazil?
Ethical approval rates for medical device trials in Brazil have improved, with compliance achieving around 75%, indicating a rising dedication to regulatory standards.
How does bioaccess® ensure data protection and client trust?
Bioaccess® is committed to data protection and has established grievance procedures to address client concerns, enhancing transparency and trust in its services.
Why is staying updated on ANVISA guidelines important for Medtech companies?
Staying updated on ANVISA guidelines is crucial for Medtech companies to successfully navigate the regulatory landscape and ensure compliance with the latest standards.