


The article delineates five pivotal strategies for effective post-market monitoring of medical devices in Latin America. It underscores the necessity of:
These strategies are underpinned by:
Collectively, these elements aim to ensure product safety and compliance within a dynamic regulatory landscape.
Navigating the intricate landscape of medical device post-market monitoring in Latin America presents both challenges and opportunities for companies operating in this dynamic region. With diverse regulatory frameworks established by entities such as ANVISA, COFEPRIS, and INVIMA, understanding these guidelines is crucial for ensuring compliance and safeguarding patient safety.
As organizations strive to enhance their monitoring strategies, the question arises: how can they effectively adapt to these evolving regulations while maintaining operational efficiency and product integrity?
This article explores five key strategies that can empower businesses to thrive in the realm of post-market surveillance, ultimately leading to improved outcomes for both patients and manufacturers.
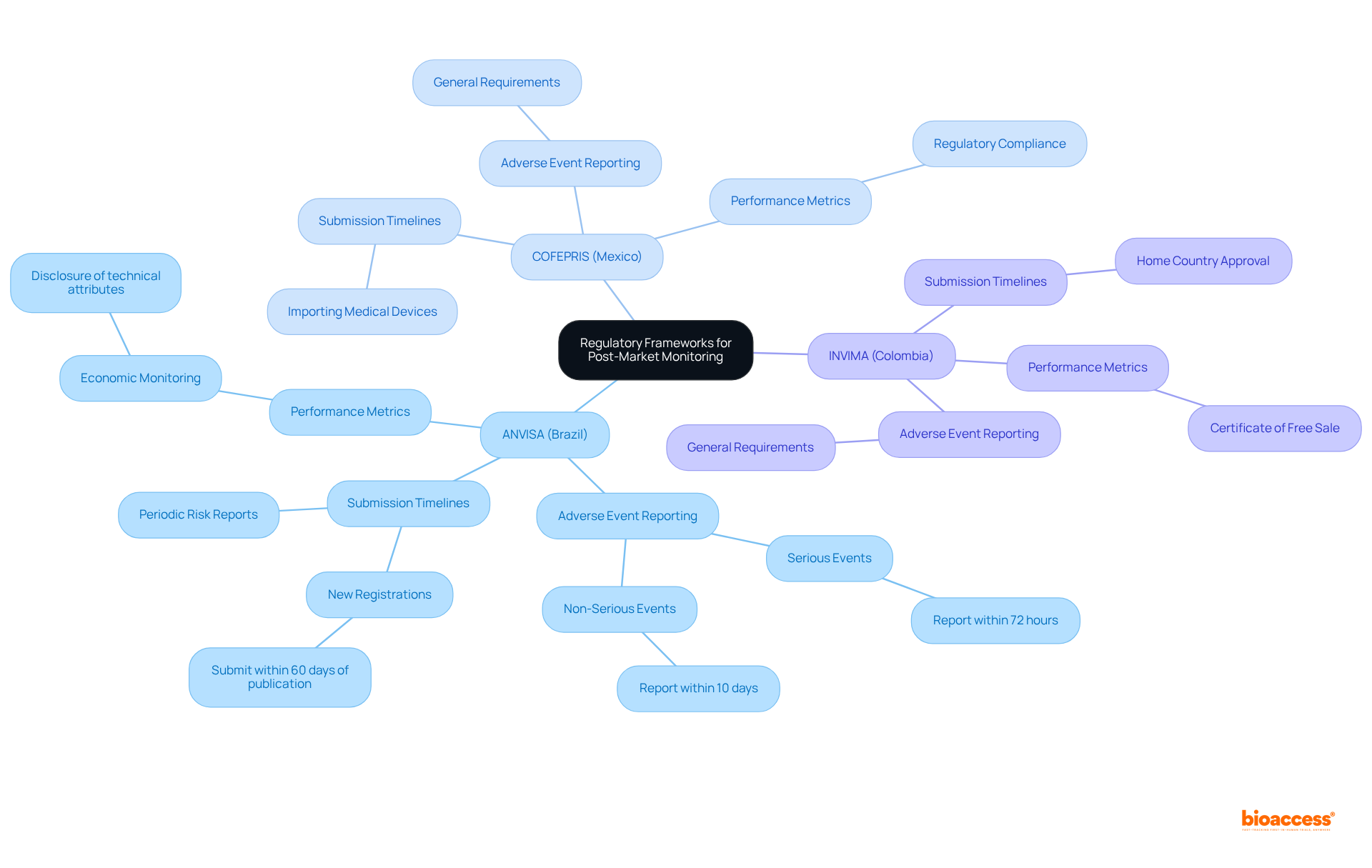
Navigating the landscape of Latin America medical device post-market monitoring requires a comprehensive understanding of the legal frameworks established by local entities, such as ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia. Each governing body enforces distinct regulations that oversee the Latin America medical device post-market monitoring of medical instruments after they enter the market.
Companies must familiarize themselves with these regulations, which include essential elements like:
For example, ANVISA mandates that adverse events leading to death or serious public health threats be reported within 72 hours, while less severe incidents must be reported within 10 days.
Engaging with local compliance specialists is crucial, as they provide insights into the intricacies of these frameworks, ensuring adherence and reducing the risk of penalties or product recalls. Additionally, staying updated on regulatory changes is vital, as these developments can significantly influence monitoring strategies and operational procedures.
By proactively adapting to these evolving regulations, companies can enhance their monitoring efforts in line with Latin America medical device post-market monitoring and maintain the safety and effectiveness of their medical products.
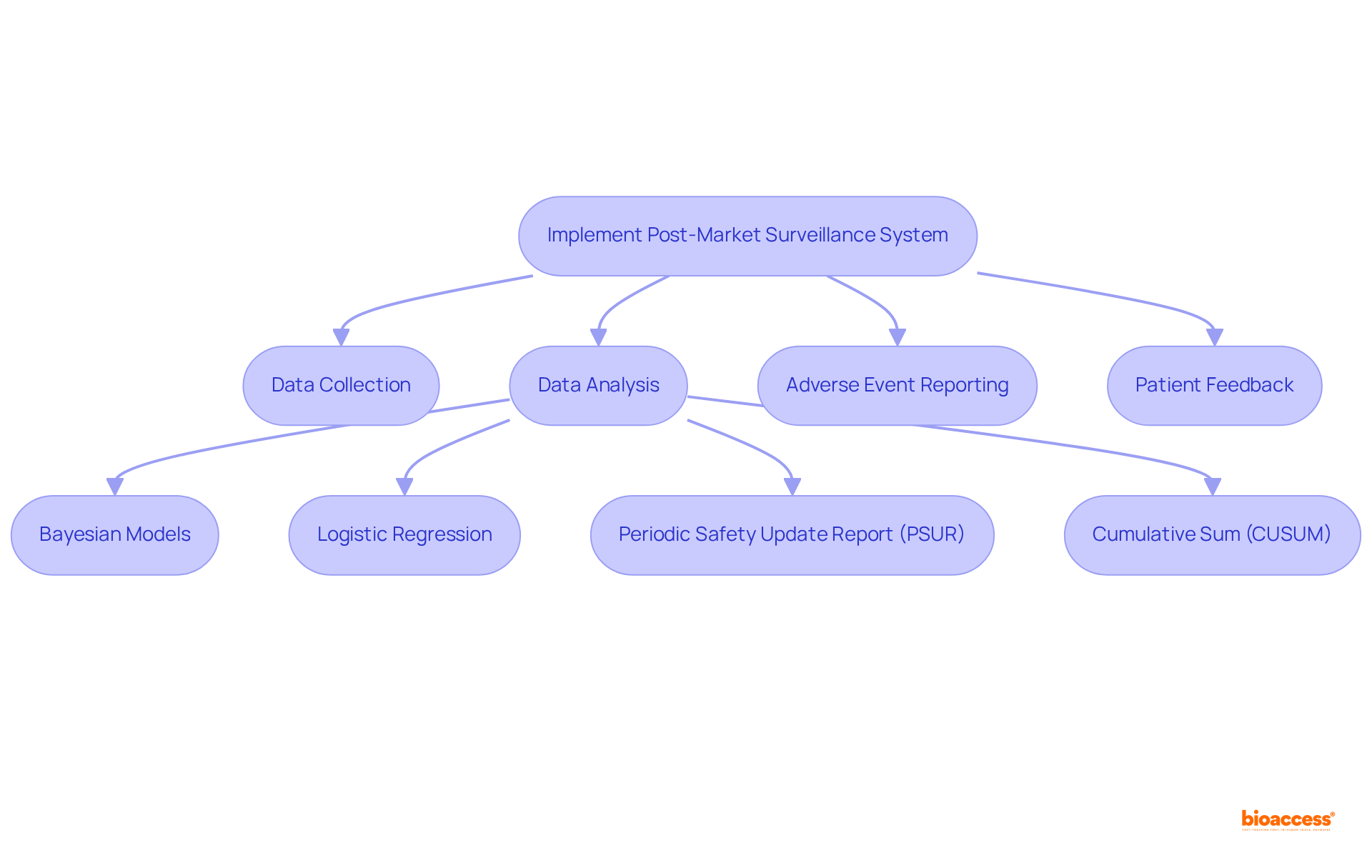
An effective system for Latin America medical device post-market monitoring is essential for ensuring the safety and performance of medical devices. It should encompass a comprehensive plan detailing how data will be collected, analyzed, and reported. This involves establishing systems for the ongoing assessment of device performance, including frameworks for adverse event reporting and channels for patient feedback.
Companies are encouraged to utilize data analytics tools, such as Bayesian models and logistic regression, to proactively identify trends and potential safety issues. The Periodic Safety Update Report (PSUR) plays a critical role in guaranteeing continuous oversight of safety and performance data, while the Cumulative Sum (CUSUM) method aids in recognizing trends in adverse events over time.
Routine audits and evaluations of the surveillance data are integral to ensuring that the system remains effective and complies with regulations, particularly under INVIMA's rigorous standards in Colombia, recognized as a Level 4 health authority by PAHO/WHO. Furthermore, integrating these systems with existing quality management processes can significantly enhance operational efficiency and responsiveness to emerging safety concerns.
As Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, emphasizes, 'Effective oversight, such as Latin America medical device post-market monitoring, is vital for maintaining product integrity and meeting compliance requirements in a continuously evolving environment.
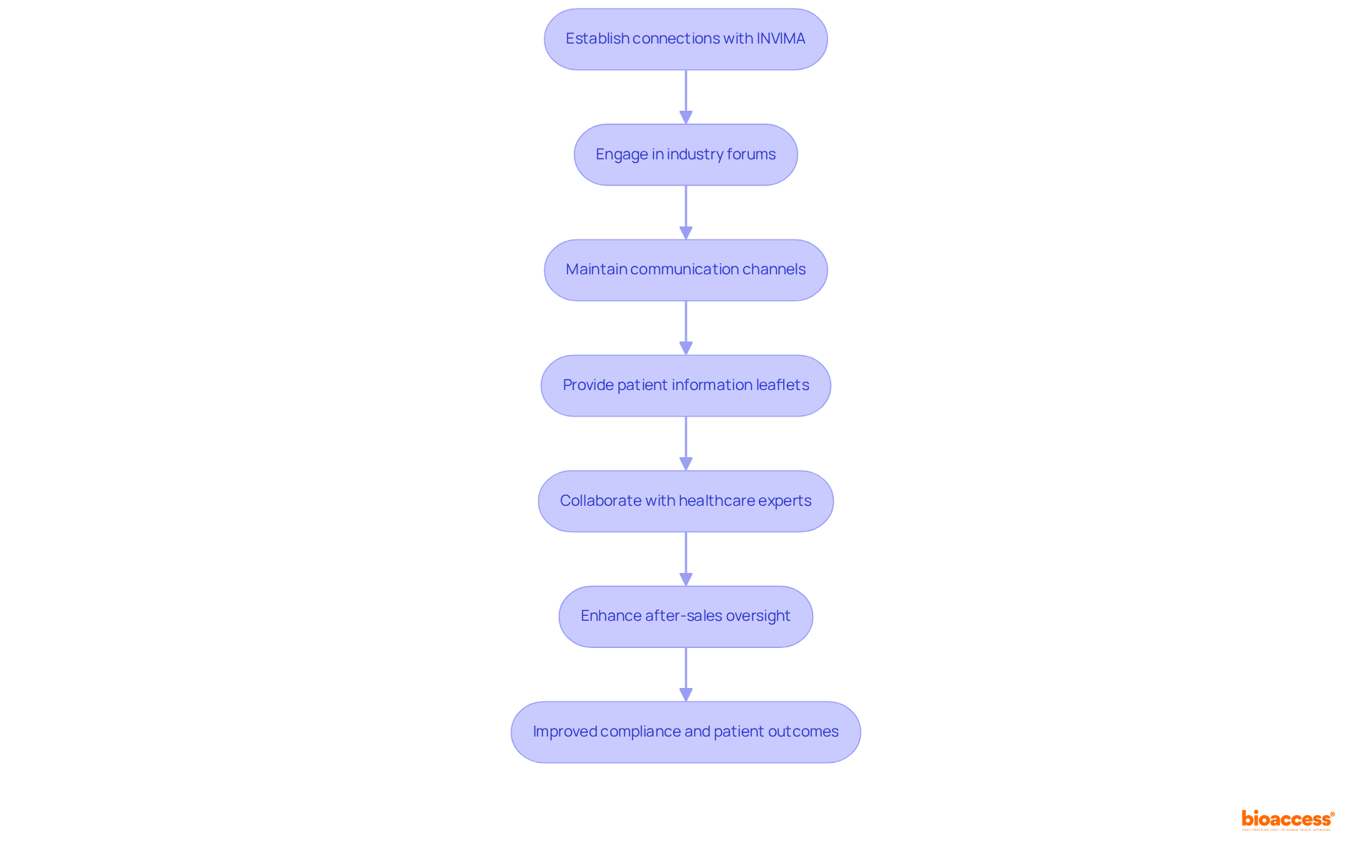
Establishing robust connections with local oversight agencies, such as INVIMA (Colombia National Food and Drug Surveillance Institute), and stakeholders is essential for effective Latin America medical device post-market monitoring. INVIMA, founded in 1992 under Colombia's Ministry of Health and Social Protection, oversees the inspection and regulation of health products, ensuring adherence to health standards. Organizations must actively engage in industry forums, workshops, and compliance meetings to remain abreast of evolving regulations and best practices. Maintaining open communication channels with INVIMA can facilitate timely responses to inquiries and swiftly address potential issues.
Moreover, recent guidelines mandate that manufacturers provide patient information leaflets and implant cards for higher-risk medical products, underscoring the critical nature of compliance in oversight efforts. Collaborating with healthcare experts, patients, and advocacy organizations yields valuable insights into equipment effectiveness and safety, thereby enhancing the evaluation process. Furthermore, INVIMA's enhancements to after-sales oversight, including increased reporting to consumers and authorities, reflect the dynamic regulatory landscape. By cultivating a collaborative environment, organizations can adeptly navigate these changes and refine their strategies for Latin America medical device post-market monitoring, ultimately contributing to improved patient outcomes and device safety. With over 15 years of experience in clinical research, bioaccess® is strategically positioned to assist companies in these vital initiatives.
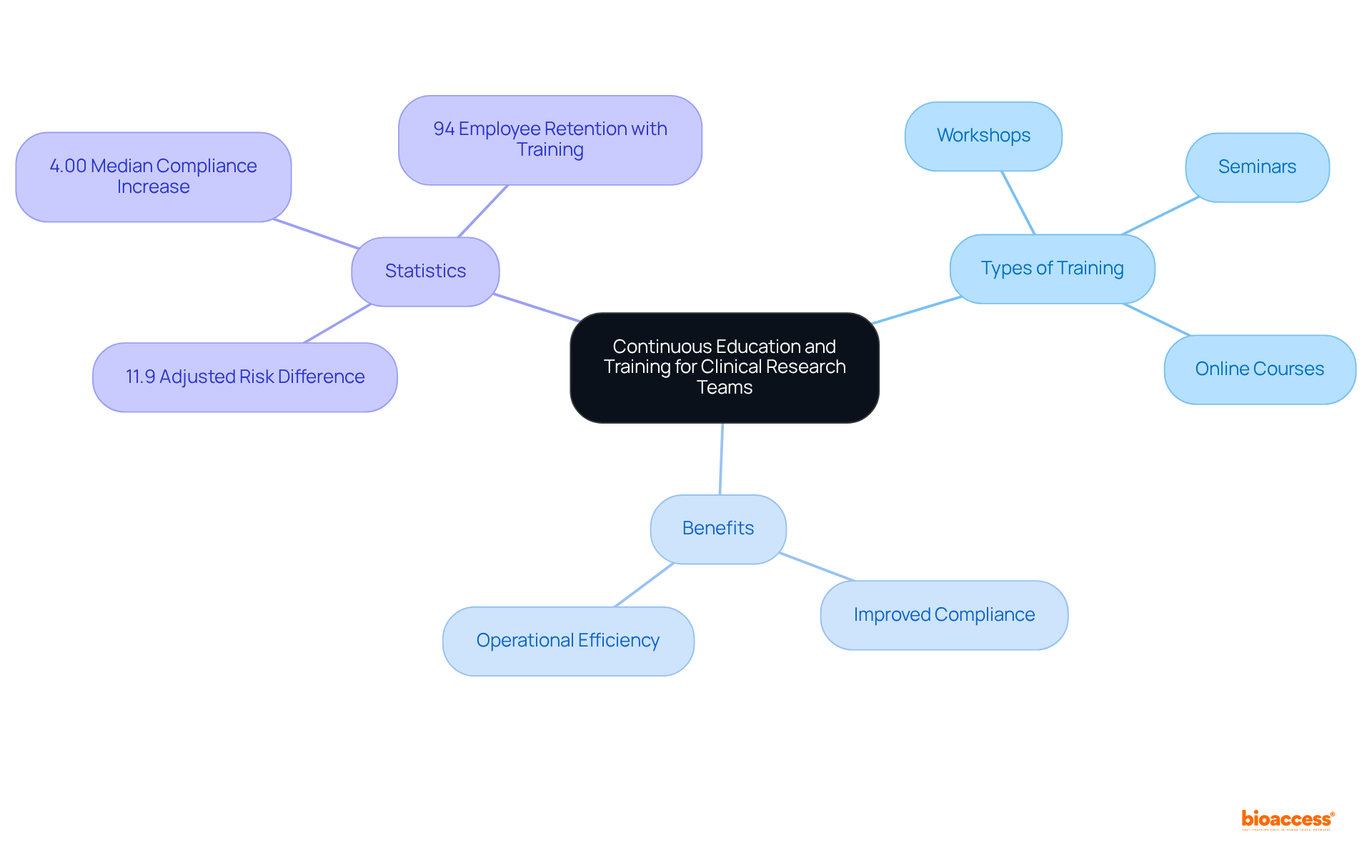
To uphold the highest standards in market surveillance, organizations must prioritize ongoing education and training for their clinical research teams. This commitment encompasses:
Statistics reveal that ongoing training significantly bolsters compliance and operational efficiency; intensive educational programs have demonstrated an adjusted risk difference of 11.9% in enhancing adherence to best practices. Encouraging team members to pursue certifications and attend industry conferences not only enriches their expertise but also ensures they remain informed about emerging trends and technologies.
By investing in the professional development of their teams, companies like bioaccess® are better equipped to confront the challenges of latin america medical device post-market monitoring, which ultimately contributes to improved patient safety and product efficacy. Successful training programs within the Medtech sector illustrate that structured educational interventions can yield a median compliance increase of 4.00%, underscoring the critical value of a well-trained workforce in navigating the complexities of latin america medical device post-market monitoring.
Furthermore, bioaccess®'s proficiency in managing feasibility studies, compliance reviews, and other clinical trial services amplifies the effectiveness of these training initiatives. Notably, a statistic indicates that 94% of employees would remain longer at a company that invests in their career development, emphasizing the crucial role of training in enhancing retention and morale.
Navigating the complexities of post-market monitoring for medical devices in Latin America is essential for ensuring product safety and compliance. Understanding the diverse regulatory frameworks established by local authorities is the first step in this intricate process. By familiarizing themselves with the specific requirements of bodies like ANVISA, COFEPRIS, and INVIMA, companies can effectively align their monitoring strategies with regulatory expectations.
The article emphasizes several key strategies, including:
Establishing robust post-market surveillance systems allows for the ongoing assessment of device performance, while collaboration with stakeholders provides critical insights that enhance compliance efforts. Additionally, investing in the education and training of clinical research teams fosters a culture of excellence and responsiveness to emerging challenges.
Ultimately, the significance of effective post-market monitoring cannot be overstated. As the landscape of medical device regulation continues to evolve, organizations must remain agile and proactive in their approaches. By adopting these best practices, companies not only safeguard patient outcomes but also contribute to the overall integrity of the healthcare system in Latin America. Embracing these strategies will ensure that medical devices not only meet regulatory standards but also fulfill their promise of improving patient health and safety.
What is the importance of understanding regulatory frameworks for post-market monitoring in Latin America?
Understanding regulatory frameworks is crucial for companies to navigate the distinct regulations established by local entities in Latin America, ensuring compliance and effective monitoring of medical devices after they enter the market.
Which local entities govern post-market monitoring in Latin America?
The main governing bodies include ANVISA in Brazil, COFEPRIS in Mexico, and INVIMA in Colombia, each enforcing its own set of regulations.
What are some key elements of the regulations for post-market monitoring?
Key elements include adverse event reporting requirements, submission timelines, and specific metrics for tracking device performance.
What are the reporting timelines for adverse events according to ANVISA?
ANVISA requires that adverse events leading to death or serious public health threats be reported within 72 hours, while less severe incidents must be reported within 10 days.
Why is it important to engage with local compliance specialists?
Engaging with local compliance specialists is vital as they provide insights into the complexities of regulatory frameworks, ensuring adherence and minimizing the risk of penalties or product recalls.
How can companies stay updated on regulatory changes?
Companies can stay updated on regulatory changes by actively monitoring developments in the regulatory landscape, as these changes can significantly impact monitoring strategies and operational procedures.
What is the benefit of proactively adapting to evolving regulations?
Proactively adapting to evolving regulations enhances monitoring efforts and helps maintain the safety and effectiveness of medical products in the Latin American market.